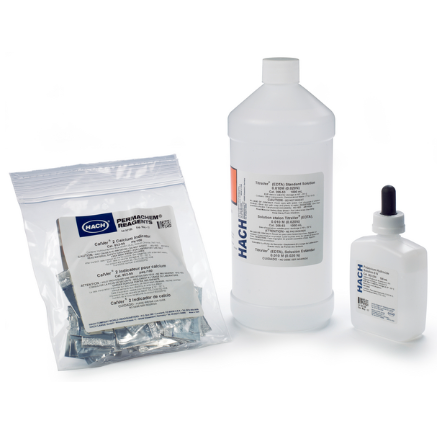
Calcium Hardness Reagent Set measures calcium hardness in water, wastewater and seawater by EDTA Titration.
Article 2447000 measures calcium hardness in the range between 0 and 5000 mg/L CaCO₃ in water, wastewater and seawater. The product uses EDTA Titration method (8222). Total Hardness Reagent Set is enough for 100 tests.
Calcium Hardness Reagent Set includes:
- CalVer 2 Calcium Indicator Powder Pillows (p/n 85299)
- Potassium Hydroxide Standard Solution, 8 N (p/n 28232H)
- EDTA Standard Solution, 0.020 N (p/n 20553)
Before you start take into account the following:
- Collect the sample in glass or plastic containers cleaned with detergent and rinsed with 1:1 nitric acid and deionized water.
- Pipet 1-2 drops of Magnesium Standard Solution, 10-g/L as CaCO₃, if the sample does not contain magnesium.
- Add 0.1-g scoop of CalVer 2 Calcium Indicator Powder as the alternative to the CalVer 2 Calcium Indicator Powder Pillow.
- Adjust the pH to 7 with Potassium Hydroxide Standard Solution before analysis.
Procedure for CaCO₃ analysis
Choose the appropriate sample volume and select the titration cartridge from the table in the working procedure description. After that pour the titrant to the 25mL buret. Fill 250mL Erlenmeyer flask with the water, wastewater or seawater sample. Pipet 1mL of 8 N Potassium Hydroxide Standard Solution in the Erlenmeyer flask and swirl to mix. Afterwards add CalVer 2 Calcium Indicator Powder Pillow and swirl the content of the Erlenmeyer flask. Place the Erlenmeyer flask under the buret, swirl the Erlenmeyer flask and continue adding titrant until the color changes from red to pure blue. Calculate the concentration (see manual for detailed information regarding calculation of the testing results).
Summary of method
Potassium hydroxide adjusts the sample pH to 12-13 that causes magnesium hydroxide precipitate to form. CalVer 2 Calcium Indicator enters into reaction with calcium giving a red color. The EDTA titrant enters into reaction with all the free calcium. After the EDTA has reacted with all of the free calcium ions, the EDTA removes the calcium from the indicator. The indicator color then changes from red to blue.
Sample preparation
In order to conserve the collected sample, adjust the sample pH to 2 or less using concentrated nitric acid. Store the conserved sample at room temperature for up to 6 months.
| Brand | Hach |
| Parameter | Calcium Hardness |
| Measuring range | 0-5000 mg/L CaCO₃ |
| Method | EDTA Titration method |
| Standard method | 8222 |
| Number of tests | 100 tests |